Welcome to the Space Physics Research Laboratory and XTRM Labs!
For over 75 years, our technicians have built electronics that operate in one of the most forbidding environments we know, with ionizing radiation from the Sun and a chill close to absolute zero. XTRM labs grew out of the Space Physics Research Laboratory (SPRL).
We’ve designed components for orbit and for landing on the moon, dust-storm-prone Mars and even a comet. But you don’t have to go to space to find these environments. Researchers operating in deserts have to put up with dust storms, those in tundra deal with frigid temperatures, and in the deep sea, pressures can reach a crushing eight tons per square inch. XTRM labs bring our space environment expertise back down to Earth. Find out how we can help you.
FEATURED NEWS
-
Space Physics Research Laboratory Moves New SPICES Instrument Closer to Realization
The new SPICES instrument developed at SPRL could accelerate particles faster than ever before onboard a spacecraft, improving their identification.…
-

Space Physics Q&A: An Interview with Quality Manager Alana Buday
Meet Alana Buday, who combined her passion for space with her skills in engineering to work as a Quality Manager…
-
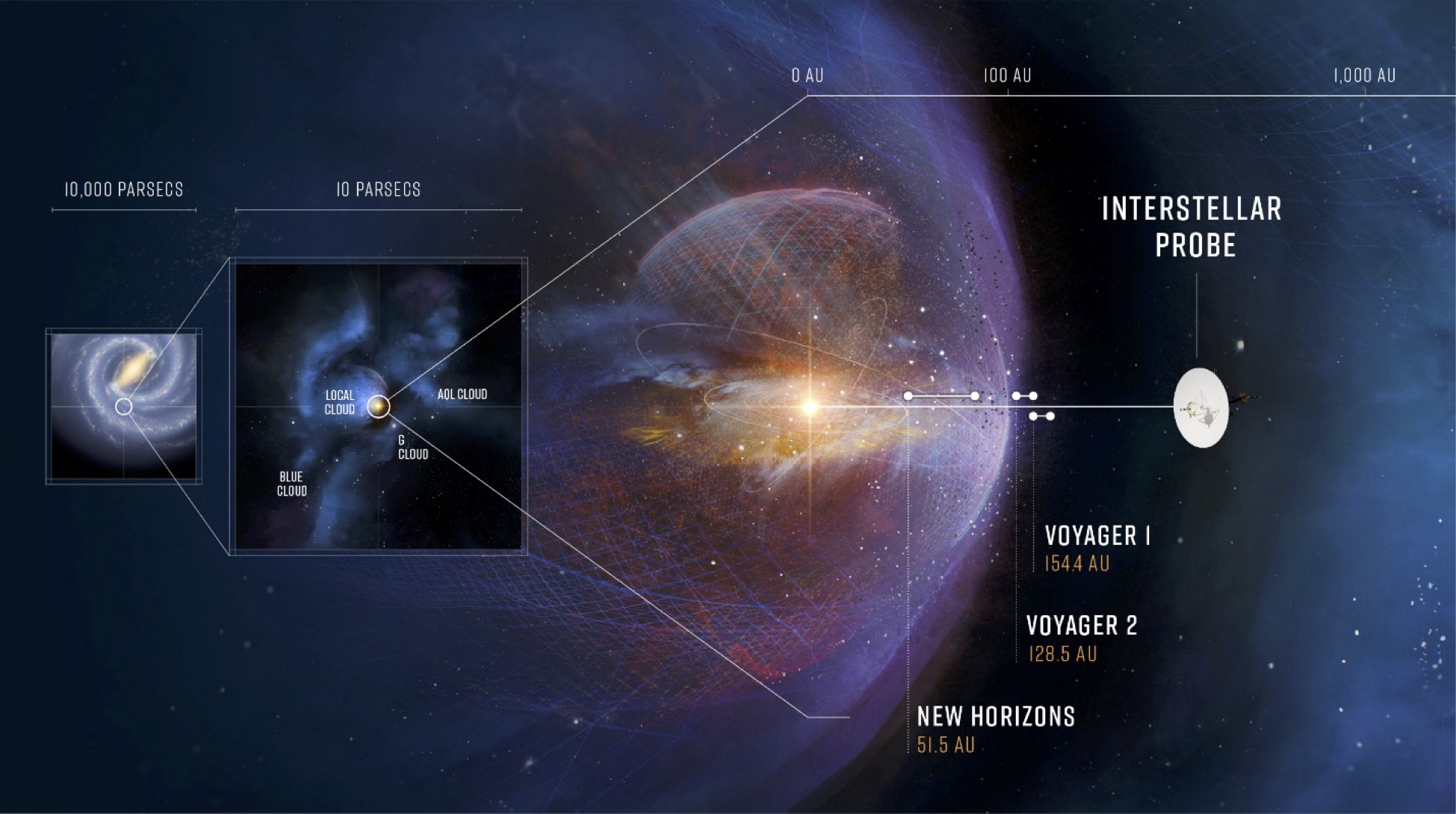
Mapping the Best Route for a Spacecraft Beyond the Sun’s Sphere of Influence
A University of Michigan-led study provides recommendations to better understand the size and shape of our home in the heliosphere.…